Research Experience & Therapeutic Areas of Interest:
- Pulmonary
- Sleep Disorders
- General Medicine
- Gastroenterology
- Smoking Cessation
- Vaccines
- Psychiatry
Research Experience (Phase I-IV)
Phase I Experience:
- First-in-Human
- SAD/MAD
- PK/ PD
- Monoclonal Antibodies/ Biologics
- Proof-of-Concept
- Bioavailabilit/ Bioequivalence
- Crossover designs
- Drug-interaction
- CYP2D6 metabolizers
- Vaccine
- Adaptive Design
- Refrigerated Centrifuge
Phase I Unit Capabilities
- 3 bed capacity
- On-site clinical laboratory
- On-site investigational pharmacy
- Holter-Monitoring
- Neuro-Cognitive testing
- EEG/ Polysomnography
- -80 degree C and -20 degree C
- Recreation areas for subjects
- On-site safe parking
Extensive Experience Facilitating Procedures and Assessments:
- Polysomnography (nPSG)
- Multiple Sleep Latency Test (MSLT)
- Maintenance of Wakefulness Test (MWT)
- Driving simulation
- Actigraphy
- CPAP Therapy
- Postural stability
- Neurological Assessments of Residual Sedation
- Vigilance Testing
- Reaction Time
- Memory Testing
- Spirometry
- Weight Loss Management
- Translation available in Arabic, Tagalog, Indonesian
Recruitable Metrics
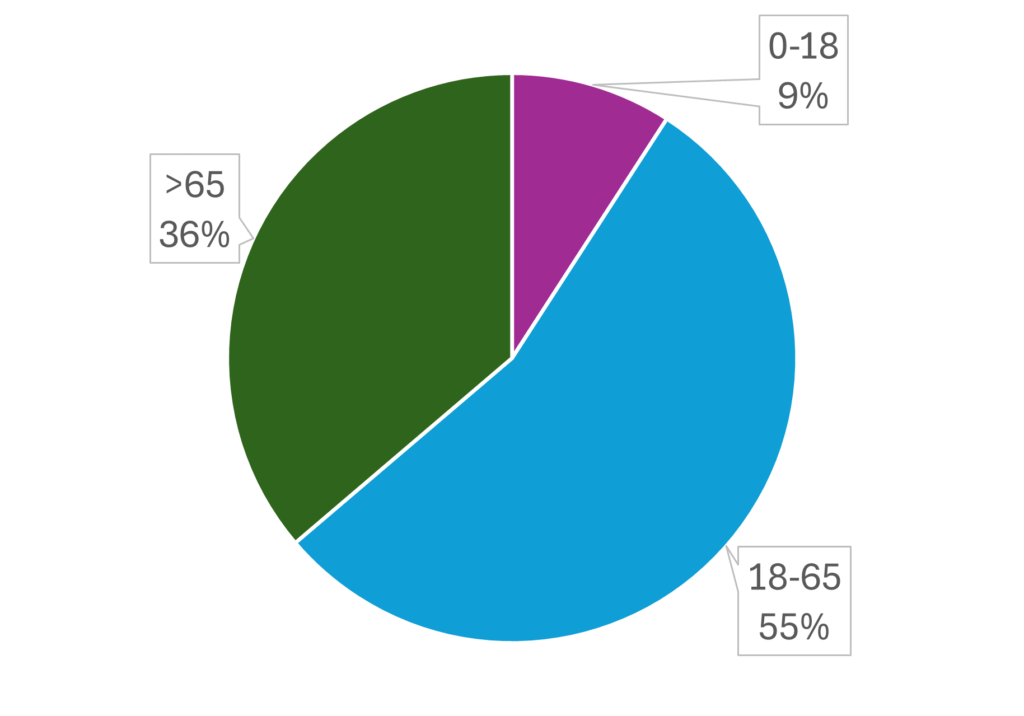
Age
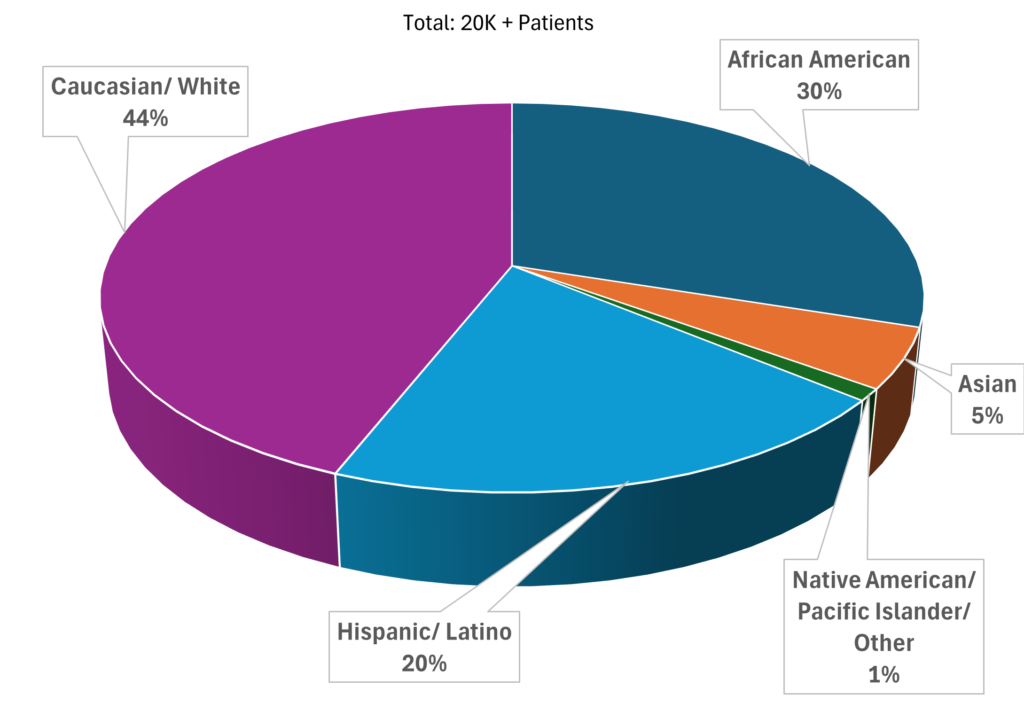
Race
Track Record



